1 Introduction
Since the introduction of the Research Governance Framework for Health and Social Care, and its subsequent replacement with the UK Framework for Health and Social Care Research 2017, it has been necessary to have in place a working policy relating to the way that Research is undertaken in Rotherham Doncaster and South Humber NHS Foundation Trust (RDaSH).
When an NHS body is the organisation providing care, it is required to confirm capability and capacity before research can begin to enable the trust to maintain records of and monitor the research taking place. Research is an essential component of developing effective health care, but it can also carry elements of risk.
These core principles are ensured through robust review of the research by the Health Research Authority (HRA), Research Ethics Committee (REC), study sponsors, funder and oversight of the conduct of the research by the Grounded Research department and researchers in the trust.
2 Purpose
This policy sets out to outline expected standards, defines individual roles and responsibilities and the processes involved in registering, adopting and gaining relevant permissions for research to begin in or involving the trust as sponsor or host. This policy sets out to ensure that the trust is fully compliant with and gives due regard to the provisions of the Research Governance Framework for Health and Social Care and the subsequent wider UK Policy Framework for Health and Care Research.
The trust will seek to do this by providing support and guidance for those who wish to undertake research. This will be done via the Grounded Research department who will enable research to be undertaken to high standards, considering ethical implications. We will support staff to understand the mandatory and Trust procedures involved in gaining approval for and conducting research within the NHS. The trust is a research active organisation that aims to promote a sustained improvement in leadership, scale, delivery and uptake of research.
As an organisation we recognise that our staff are our greatest asset and we are committed to developing a culture whereby research is embedded as a core part of clinical services, enhancing our offer to those who access our services, but also making the trust an excellent place for staff to work, learn and innovate.
In summary, it is trust policy to:
- maximise the opportunities available for staff, service users and carers to take part in, and lead high quality clinical and health research
- prioritise support for National Institute for Health and Care research (NIHR) portfolio research as the first call on resources
- encourage clinical service management to consider research at all relevant meetings and consider staff requests for time and other resources transparently and in good faith considering research to be core business of the trust
- protect the safety, dignity, rights and well-being of all patients and staff involved in clinical and nonclinical research
- abide by the human resources good practice guidance for research (research passport scheme)
- ensure that arrangements are in place for the management and monitoring of clinical trials and all other research studies where the trust has taken on the role of sponsor, as a host, or a collaborator institution, including compliance with all applicable laws and regulations.
- the trust will still strive to meet the former National Institute for Health and Care Research (NIHR) benchmarks for performance in initiation (time in days to approve valid applications); for first patient first visit (FPFV); to strive towards first global; first European; first UK patient recruited, and to recruit to time and target to remain an attractive site to conduct research
- encourage all research outside the NIHR portfolio to comply with and perform to similar performance metric levels and benchmarks
- conduct assess, arrange, and confirm (AAC) procedures and other mandatory processes using a consistent yet flexible, risk-proportionate approach, delegated to be conducted by the Grounded Research department combined with robust feasibility to ensure timely approval and delivery of sponsored and hosted research with proportionate oversight and delegation of responsibility
- consider that conducting research outside of internal, national and international regulatory and approval frameworks is considered serious misconduct and would justify disciplinary action, up to and including dismissal in the worst cases
3 Scope
- This policy applies to anyone conducting or taking part in research within the trust, irrespective of whether such research is sponsored or hosted by the trust.
- This policy applies to all areas of the trust, and all employees of the trust, including individuals employed by a third party (operating under a letter of access or honorary contract), by external contractors, as voluntary workers, as students, as locums or as agency staff.
4 Responsibilities, accountabilities and duties
4.1 Responsibilities
The chief executive has overall responsibility for the strategic direction and operational management of the trust.
The medical director has responsibility for identifying, developing and implementing a research governance policy that supports the chief executive in their responsibility.
It is the responsibility of the research governance manager to draft, implement and update this policy.
It is the responsibility of the Research and Innovation group and Grounded Research Senior Management team to approve this policy.
It is the responsibility of the research and innovation manager to ensure the Grounded Research department works with colleagues across the trust to embed this policy in daily practice.
It is the responsibility of the research governance manager to ensure that the policy and associated procedures are followed within the Grounded Research department.
It is the responsibility of all trust staff to identify the need for any change to this policy when becoming aware of changes in practice, changes to statutory requirements, revised professional or clinical standards and local or national directives, and advising their line manager accordingly.
4.2 Research and innovation group
The Research and Innovation group is accountable and responsible for decisions made in the following four domains:
- oversight of the research and innovation plan
- ensuring research excellence is sufficiently considered for all 28 promises
- responsibility for resolving cross-trust issues pertinent
- delegated responsibility from the clinical leadership executive (CLE) for specific areas of delivery, including approval of relevant trust-wide policies
4.3 Research and innovation panel
The research and Innovation panel supports the research governance function and reviews, approves and monitors all research projects in the trust (see appendix A).
4.4 Delegation of responsibilities
Authorisation for all research projects lies with the research and innovation panel. Other duties are delegated as follows:
| Task or process | Designated officers |
|---|---|
| Promoting a positive culture of research within the trust | All |
| Functioning as the first point of contact for research within the trust | All |
| Taking action if misconduct or fraud is suspected, in line with the trust counter fraud, bribery and corruption policy and the disciplinary policy | Director of research and innovation group |
| Alerting the medical director or director of research and innovation of any complaints received related to research activity and supporting investigation as agreed at the research and innovation panel | Director of research and innovation group |
| Supporting access to appropriate expertise in the design of research | Research and innovation group manager |
| Supporting the planning and delivery of research | Research and innovation group manager |
| The management, development and implementation of research governance systems and procedures | Research governance manager |
| Highlighting changes to legislation and national processes relating to research, and ensuring these changes are implemented across the organisation | Research governance manager |
| Making researchers aware of governance requirements and ensuring they understand their responsibilities | Research governance manager or research and innovation group manager |
| Providing support via access to information on research governance procedures and regulatory requirements | Research governance manager |
| Advising and assisting researchers through the requirements of the research approvals processes, including Health Research Authority, Research Ethics Committee, and Medicines and Healthcare products Research Agency as required | Research governance manager |
| Ensuring that all applicable regulatory requirements are adhered to | Research governance manager or research and innovation group manager |
| Ensuring that research is conducted in accordance with good clinical practice principles | Research governance manager |
| Reviewing capability and capacity of all research taking place including obtaining internal authorisations | Research governance manager |
| Ensuring that research is well managed, monitored and reported | Research governance manager or research and innovation group manager |
| Ensuring that the conduct of trust sponsored studies are appropriately monitored | Research governance manager or research and innovation group manager |
| Monitoring recruitment to time and target and providing support or advice where appropriate | Research governance manager |
| Communicating, research summaries or appropriate reports to a variety of audiences | Research governance manager or research and innovation group manager |
| Alerting the medical director or director of research and innovation of any complaints and supporting investigation as agreed at the research and innovation panel | Deputy director of research and innovation and research and innovation manager |
| Managing research passport validation for trust employed staff conducting research outside the organisation | Research governance manager |
| Ensuring that honorary research contracts and letters of access are issued for external researchers coming into the organisation | Research governance manager |
| Managing research passport validation for trust employed researchers and research support researchers and research support staff conducting research outside the organisation | Research governance manager |
4.5 Researcher responsibilities
Each researcher is accountable for their own practice and for abiding by the UK Framework for Health and Social Care Research, good clinical practice and other relevant legislation and regulations relating to research that they are involved with.
The researcher is responsible for:
- notifying the Grounded Research department of research they are planning to undertake by submitting the research registration form (see registering a research project (non-portfolio) procedure) and submitting the local information pack as defined by the Health Research Authority (where Health Research Authority approval is required)
- adhering to the approved research protocol.
- complying with legal requirements and obtaining the prior approval of the relevant care group and, or director research and innovation
- provide information to the appropriate managers and clinical staff regarding research being conducted in their service, department, or ward, this should include information regarding the conduct of the research and especially anything relevant to the participant’s wellbeing
- safeguarding participants’ welfare whilst in the study
- retain responsibility, where applicable, for research participants’ care
- the dissemination and publication of their work, with the guidance of the Grounded Research department.
- adhering to all trust policies and procedures, reporting any adverse incidents connected to the research in line with the trusts incident management procedures (see incident management policy) and the incident reporting system (IR1). In addition, specific legal requirements exist to report incidents, for example, adverse reactions to the Medicines and Healthcare products Regulatory Agency
- adhering to the Health and Safety at Work Act, (see health and safety policy) in respect to themselves and their participants
- all those involved in research with human participants, their organs, tissues or data, must be aware of and implement the basic principles from the UK Framework for Health and Social Care Research
- the appropriate use and protection of patient information is of paramount importance to the trust, all individuals involved in research must be aware of their legal and ethical duties and particular attention must be given to systems for ensuring confidentiality of personal information and to the security of these systems
- all relevant trust policies and procedures must be adhered to, for example, information governance policy and management framework, which includes data protection, healthcare record keeping policy and records management policy and the requirements of the Caldicott guardian
- research material such as questionnaires or raw data must be kept in accordance with NHS guidance (see records management policy for retention periods)
- all those carrying out research to which the requirements of the Mental Capacity Act (2005) policy, apply must act in accordance with the provisions of the act
4.6 Competence
All staff employed by the trust for the sole purpose of undertaking research roles must be able to demonstrate an understanding of current national and local policies and procedures.
All staff undertaking research activities in line with the scope of this policy must be able to demonstrate that they have received suitable training relevant to their identified roles and responsibilities.
All external researchers wishing to work with the trust for the purpose of data collection must confirm upon application that they have the required knowledge and expertise to complete the requested tasks. This will form part of their contract with the trust.
Individuals working on clinical trials of investigational medicinal products (CTIMPs) study will need to have completed good clinical practice (GCP) training or update within the last 2 years. Other individuals will need to complete this training within the last two years unless GCP training requirements are agreed otherwise, for example for some roles GCP fundamentals might be sufficient. Refer to the good clinical practice (GCP) for research procedure.
5 Procedure
5.1 Health Research Authority approval and research and development confirmation of capacity and capability
5.1.1 Health Research Authority approval
The dignity, rights, safety and wellbeing of participants must be the primary consideration in any research study. The Department of Health and Social Care requires that research involving patients, service users, care professionals or volunteers, or their organs, tissue or data is reviewed independently to ensure it meets ethical standards. The Health Research Authority (HRA) and Health and Care Research Wales (HCRW) approval is the process for the NHS in England and Wales that brings together the assessment of governance and legal compliance with the independent Research Ethics Committee opinion. It replaces the need for local checks of legal compliance and related matters by each participating organisation.
All research projects taking place within the NHS must have Health Research Authority approval, and any other approvals depending on the study type, before any research activity can commence. The Health Research Authority provides the guidance on the approvals required for different types of research studies, please see what approvals and decisions do I need.
5.1.2 Research Ethics Committee favourable opinion
The Research Ethics Committee (REC) is not accountable in any way to NHS Trusts, and in particular is separate from Trust Research Departments in respect of the accountability for their operational processes and decision-making.
The Research Ethics Committee is part of the Health Research Authority assessment and is only required for studies including service users, patients and carers, for studies involving NHS staff only REC is not needed.
All studies must have appropriate arrangements for gaining consent. Care is needed for obtaining consent from children and vulnerable adults such as those with mental health problems or learning disabilities. Consent processes are reviewed by the Research Ethics Committee.
Where ethical approval is obtained for research with participants lacking capacity to consent, the requirements of the Mental Capacity Act (2005) must be followed. This process allows the trust Research and Development team to focus on assessing, arranging and confirming the capacity and capability to deliver a study.
5.1.3 Confirmation of capacity and capability
Confirmation of NHS trust capacity and capability is the local feasibility procedure undertaken by an NHS organisation to assess and confirm whether the organisation has the resources, policies and service users required to successfully deliver the research study to time and target (see appendix D)
The research governance manager will ensure that the process of research confirmation is as streamlined as possible, and work with corporate departments to deal with barriers in the system which delay opening studies.
The Research Governance team will follow national Health Research Authority guidance to assess, arrange and confirm the trusts’ ability to run a study.
Once research approvals are in place, significant changes or developments to research proposals, such as change in protocol, must be communicated to the Health Research Authority, through a notice of substantial amendment (see Health Research Authority amending approval for details). The amendment is applied for under the amendment tab on the integrated research application system (IRAS) page. The notice of substantial amendment should be emailed to the Research Ethics Committee (REC). With regard to a non-substantial amendment you are required to notify the NHS or health and social care participating organisations and use the template form. More information can be found via Health Research Authority amending approval.
The trust has an established organisational structure for managing research and innovation. The research and innovation panel provides assurance to the Research and Innovation group that all research projects have adequate consideration of:
- sponsorship
- ethical review
- scientific review
- evidence of funding
- safety of participants
- researchers and other staff
- trust resource requirements
- data protection and intellectual property
5.2 Scientific review
All existing sources of evidence must be considered carefully before undertaking research. Research which duplicates other work unnecessarily, or which is not of sufficient quality to contribute something useful to existing knowledge, is unethical.
Every proposal must be subjected to review by experts in the relevant field able to offer independent advice on its quality. It is the research sponsor’s responsibility to ensure adequate peer review is in place which is proportional to the scale of the research. For example:
- portfolio research has been peer reviewed as part of the adoption process
- externally funded research (for example, from a research council or charity), it is expected that peer review would have been undertaken as part of the application process
- commercial sponsored projects, it is the responsibility of the commercial sponsor to arrange peer review
- student projects, the peer review processes of the university involved should normally be adequate
- self-funded research where the trust is to act as the research sponsor, the Grounded Research department will help to arrange an independent peer reviews
- evidence of peer reviews must be in place before applying for Health Research Authority approval
- the research protocol must be submitted to the research and innovation panel to check that the project fits with the research strategy and is feasible to run in the trust
5.3 Study agreements and contracts
Before a piece of research can start, sponsors and host institutions need to have an appropriate agreement in place which set out the responsibilities of the parties involved in research. The UK Clinical Research collaboration (UKCRC) and stakeholders have developed a suite of model agreements as follows:
- commercial clinical trial of an investigational medicinal product (CTIMP) studies, commercial companies are expected to use the national model clinical trial agreement (model clinical trial agreement (mCTA) or clinical research organisation model clinical trial agreement (CRO mCTA)) for pharmaceutical companies working with the NHS
- commercial studies involving medical devices, commercial companies are expected to use the national model devices clinical trials agreements (devices mCTA)
- non-commercial studies, non-commercial partners are expected to use the national non-commercial clinical trial agreement (mNCA)
Please refer to the research sponsorship procedure for further information.
Appropriate employment arrangements must also be in place for research staff. For NHS staff, evidence of their employment status will be required. Researchers not employed by any NHS organisation and requiring access to the trust will be required to complete a research passport form, and when completed send to the research governance manager. It is the responsibility of the chief investigator or principal Investigator to ensure staff have the necessary contracts or letters of access in place before staff begin research work within the trust.
Please refer to the research passport, honorary collaboration contract, honorary research contract, letter of access procedure for further information.
5.4 Risk assessment and management
Research can involve increased risks arising from the research activity as opposed to the baseline level of risk arising from normal clinical practice. Risk should be assessed during protocol development to manage risk with patient autonomy and safety as paramount concerns. Risk will be controlled by systems in place to ensure that:
- projects have a sponsor
- projects are peer-reviewed
- projects are approved by the Health Research Authority
and confirmed by research and development - research proposals are taken through a staged approach of approval before the research can commence
- sponsors and Researchers act within the UK Policy Framework for Health and Social Care Research
- staff have appropriate training
- research is appropriately audited and monitored
Please refer to the research sponsorship procedure for further information.
5.5 Assess arrange confirm, confirmation of capability and capacity
All research must follow the procedures outlined below. Research activities must not start at site until the Grounded Research department provides confirmation of capability and capacity (CC&C). Approval is dependent upon:
- all research projects involving human participants, their data or tissue samples, must have Health Research Authority approval or appropriate exemption.
- research Ethics Committee favourable opinion is required except for studies deemed exempt by Health Research Authority
- for research that requires significant resource to be provided by the trust, approval should be obtained from the relevant care group director (or delegated individual) as well as the service manager (or equivalent manager, band 8 or above)
- appropriate approvals from the care group and service manager:
- where there is no appropriate band 8 manager (for example they are involved in the research) the approval should be escalated to the next level of management.
- support service approval must be obtained where the service is required to support any form of research activity, including but not limited to pharmacy, contracts, finance or information governance
- any research involving medicines must go through the trust Medicines Management Committee for approval
- research involving new processes using patient identifiable information, for example, patient names, addresses, dates of birth on a database or spreadsheet must have a data protection impact assessment approved by the trust data protection officer (see information governance policy and management framework)
5.6 Finance and intellectual property
All researchers must comply with the procedures of trust standing financial instructions in planning and accounting for all expenditure.
All researchers must be aware of and adhere to the counter fraud, bribery and corruption policy).
Where there is any potential intellectual property identified researchers must comply with the trust’s intellectual property exploitation policy.
Where the research is externally funded, the findings will be subject to contractual agreement with the research funder and, or the employing organisation before commencement. The trust must ensure that there are agreements between them and research funders or other care organisations about ownership, exploitation and income from any intellectual property that may arise from research conducted by their employees.
5.7 Involvement of consumers, service users, carers and the public in research
Public involvement in research means research that is done with or by the public, not to, for or about them. It means that patients or other people with relevant experience contribute to how research is designed, conducted and disseminated. The Health Research Authority has guidance about public involvement. Participants, or their representatives, should be involved wherever possible in the design, conduct, analysis and reporting of research.
The trust has a group of patient research ambassadors (PRA) who are patients, carers, members of the public and people who have taken part in research before, as well as those who haven’t. They promote and support public and professional engagement in research. They volunteer their time to help spread the word about health and care research to patients and the public, especially groups who are currently less likely to take part in research.
Once established, research findings must be made available to those participating in the research (including the relatives of deceased patients who have consented to the use of tissue in the research) and to all those who could benefit from them, through publication and, or other appropriate means.
As health and social care research is conducted for the benefit of patients, users, care professionals, and the public in general, there should be free access to information both on the research being conducted and on the findings of the research, once these have been subjected to appropriate scientific review. Reports will be comprehensible and consider language and special requirements.
5.8 Complaints and misconduct
Where a complaint is made relating to research taking place in the trust, the complaint will be thoroughly investigated by the director of research and innovation or deputy director of research and innovation with support from the Grounded Research senior managers. The incident and investigation are reported to the next research and innovation panel and, if appropriate, action will be taken in accordance with the human resources disciplinary policy. Where appropriate the trust incident reporting process will be followed by submitting a report in the incident reporting system.
If the director of research and innovation or deputy director of research and innovation deems it necessary, the research and innovation panel will hold an emergency meeting to implement an immediate investigation into the possible research misconduct. Depending on the findings of the investigation actions will be agreed. These may include actions to improve protocol compliance, requiring the research team to complete additional training and, or introduction of an improvement plan.
5.8.1 Serious breaches
The clinical trials regulations require the reporting of serious breaches of good clinical practice or the protocol (regulation 29A of S12004-1031 as amended). Guidance on the definition and reporting requirements can be found on the Medicines and Healthcare products Regulatory Agency Serious Breach and Good Clinical Practice Reporting. In the UK, serious breaches should also be reported to the relevant ethics committee at the same time as the report to the Medicines and Healthcare products Regulatory Agency, in accordance with Research Ethics Committee standard operating procedures.
Good clinical practice and serious breach reporting is a legal requirement which is relevant to all trials. Where the findings identify a serious protocol breach the research can be suspended or closed at site in line with good clinical practice. Professionals may be subject to disciplinary action in with the event of a serious breach of good clinical practice.
Each professional group is also responsible to their respective regulatory body, such as the General Medical Council (GMC), or the Nursing and Midwifery Council (NMC).
5.8.2 Urgent safety measures
Good clinical practice must be adhered to at all times, as part of these requirements for safety reporting to regulatory bodies will be followed. The procedures for reporting urgent safety measures (USM) will vary depending on the type of study and whether it is sponsored or hosted by the trust but must always be reported to the research and innovation panel.
Any urgent safety measures relating to a clinical trial of an investigational medicinal product (CTIMP) should be communicated to the sponsor immediately. The sponsor must notify the Medicines and Healthcare products Regulatory Agency within 24 hours. Further details can be found on Medicines and Healthcare products Regulatory Agency.
For non-CTIMP research, the chief investigator must notify the sponsor and the Research Ethics Committee immediately of any urgent safety measure and in any event within three days.
Further details are available in the clinical trials toolkit.
5.9 Indemnity
The organisation or partnership that takes on overall responsibility for proportionate, effective arrangements being in place to set up, run and report a research project, is the sponsor. All health and social care research should have a sponsor. This includes all research that involves NHS patients, their tissue or information. All research must have a nominated sponsor who will carry non-negligent indemnity. Further information is available from the Health Research Authority roles and responsibilities.
With regard to indemnity, agreements will be documented that include the responsibilities of all parties involved within the study
This may include where there is:
- work at more than one trust site
- trust staff working at another trust
- external staff working at the trust under an honorary contract or letter of access
- researchers employed by more than one organisation
- patients, users and care professionals from more than one organisation
- more than one source of funding
For research led by trust employed staff, the trust can act as sponsor and provide full indemnity for that research study, providing the research is not for an academic qualification (see sponsor policy).
Researchers not employed by the trust but accessing the trust resources for the purposes of their research will have to provide evidence of indemnity. This is usually provided as part of the research passport process (see research passport, honorary collaboration contract, honorary research contract, letter of access procedure).
Any researcher employed by the trust who does not follow the agreed process for approval of their research will not be entitled to trust indemnity.
Where the approved study protocol is not adhered to the trust, as sponsor organisation, can suspend or terminate a study following investigation by the research and innovation panel.
5.10 External organisations
Where research is externally funded, the funding organisation has the responsibility for ensuring that the funds are used in the manner that they are intended.
Where research is externally sponsored, the sponsoring organisation has the responsibility for ensuring that sponsorship responsibilities are met, the sponsor being legally responsible for the conduct of the research.
In the event that a participant makes a complaint to the trust, the listening and responding to concerns and complaints policy should be followed and where appropriate the sponsor should be informed of the event only in a fully anonymised report. Research participants should be able to notify the sponsor, trust or both of any complaint they may have. Study specific contact details must be provided.
5.11 External researchers
External researchers, without either a clinical contract or student placement with supervision, who wish to conduct their research on trust premises, access patients, and, or patient data must have an honorary contract or a letter of access. The research passport system (see research passport, honorary collaboration contract, honorary research contract, letter of access procedure) and associated procedures have been developed to ensure that the interests of all parties are considered and the safety of patients and are maintained. Furthermore, these procedures have been developed in parallel with other arrangements across the UK to streamline the processes for obtaining permission from NHS organisations to undertake research.
- A research passport can be used as evidence with which to apply for an honorary contract or letter of access.
- National arrangements are outlined within the research in the NHS human resources good practice resource pack.
- Trust internal arrangements between human resources and the Grounded Research department allow the Grounded Research department to manage the processes.
6 Training implications
As a trust policy, all staff need to be aware of the key points that the policy covers. Line managers are responsible for all staff they manage being aware of how they can access copies of this policy.
There are no specific training needs in relation to this policy; however anyone involved in research will need to be familiar with its contents.
Staff involved in research delivery may need to have current good clinical practice training (within the last two years). The Grounded Research department can confirm if required, and the level of training, required and provide details of providers (see good clinical practice (GCP) for research procedure).
Researchers and research support staff will be made aware of this policy through one-to-one meetings or supervision and as part of the confirmation of capability and capacity process which highlights the need to adhere to trust policies.
7 Monitoring arrangements
Grounded Research is responsible for informing the trust board of all significant developments, risks, and progress. Bi-annual updates are submitted to research and innovation group which is a sub-committee of the trust’s board of directors. Reporting is managed by the research and innovation panel. The medical director or research and innovation director provides the research and innovation group with an annual end of year report (November) and a six-month assurance report (May) each year. Any other issues are recorded via exception reporting.
7.1 Research projects
- How: Use of audit tool. A minimum of 10% of open research projects.
- Who by: Grounded Research team.
- Reported to: Research and innovation panel.
- Frequency: Annually.
7.2 National Institute for Health and Care Research portfolio studies
- How: EDGE database, and open data platform reports.
- Who by: Grounded Research team.
- Reported to: Research and innovation panel and Yorkshire and Humber Regional Research Delivery Network.
- Frequency: Monthly.
7.3 Register of research
- How: EDGE database, and open data platform reports.
- Who by: Grounded Research team.
- Reported to: Research and innovation panel.
- Frequency: Monthly.
7.4 Research study activity
- How: Recruitment reports, and regular contact with local and central study teams.
- Who by: Grounded Research team.
- Reported to: Research and innovation panel.
- Frequency: Monthly.
7.5 Research activity in trust
- How: Annual end of year report and six-month assurance report.
- Who by: Research director.
- Reported to: Research and innovation group.
- Frequency: Bi-annually.
8 Equality impact assessment screening
To access the equality impact assessment for this policy, please email rdash.equalityanddiversity@nhs.net to request the document.
8.1 Privacy, dignity and respect
The NHS Constitution states that all patients should feel that their privacy and dignity are respected while they are in hospital. High Quality Care for All (2008), Lord Darzi’s review of the NHS, identifies the need to organise care around the individual, “not just clinically but in terms of dignity and respect”.
As a consequence the trust is required to articulate its intent to deliver care with privacy and dignity that treats all service users with respect. Therefore, all procedural documents will be considered, if relevant, to reflect the requirement to treat everyone with privacy, dignity and respect, (when appropriate this should also include how same sex accommodation is provided).
8.1.1 How this will be met
No issues have been identified in relation to this policy.
8.2 Mental Capacity Act (2005)
Central to any aspect of care delivered to adults and young people aged 16 years or over will be the consideration of the individuals’ capacity to participate in the decision-making process. Consequently, no intervention should be carried out without either the individual’s informed consent, or the powers included in a legal framework, or by order of the court.
Therefore, the trust is required to make sure that all staff working with individuals who use our service are familiar with the provisions within the Mental Capacity Act (2005). For this reason all procedural documents will be considered, if relevant to reflect the provisions of the Mental Capacity Act (2005) to ensure that the rights of individual are protected and they are supported to make their own decisions where possible and that any decisions made on their behalf when they lack capacity are made in their best interests and least restrictive of their rights and freedoms.
8.2.1 How this will be met
All individuals involved in the implementation of this policy should do so in accordance with the guiding principles of the Mental Capacity Act (2005) (section 1).
9 Links to any other associated documents
- Listening and responding to concerns and complaints policy
- Records management policy
- Counter fraud, bribery and corruption policy
- Data Protection Act (2018) and research procedure
- Delegation of study duties procedure
- Disciplinary policy
- Document version control and research procedure
- Healthcare record keeping policy
- Health and safety policy
- Incident management policy
- Information governance handbook
- Information governance policy and management framework
- Intellectual property exploitation policy
- Investigator site file procedure
- Performance capability management policy and procedure
- Registering a research project (non-portfolio) procedure
- Research passport, honorary collaboration contract, honorary research contract, letter of access procedure
- Standing financial instructions
- Good clinical practice (GCP) for research procedure
10 References
- UK Framework for Health and Social Care Research (HRA and Department of Health published October 2017)
- Mental Capacity Act, Health Research Authority
- National Institute for Health Research (2010) Research in the NHS. HR Good Practice Resource Pack. Human resources good practice: Information for researchers, research and development and human resources at higher education institutions and the NHS
- National Institute for Health Research Clinical Trials Toolkit
- The Medicines for Human Use (Clinical Trials) Amendment Regulations (2006)
11 Appendices
11.1 Appendix A definitions and explanations of terms used
| Acronym | Long form |
|---|---|
| AAC | Assess arrange and confirm |
| CC and C | Confirmation of capability and capacity |
| DBS | Disclosure and barring service |
| DDR and I | Deputy director of research and innovation |
| DR and I | Director of research and innovation |
| DHSC | Department of Health and Social Care |
| GCP | Good clinical practice |
| HRA | Health Research Authority |
| IRAS | Integrated research application system |
| LIP | Local information pack (trial documents) |
| MHRA | Medicines and Healthcare products Regulatory Agency |
| NIHR | National Institute for Health and Social Care Research |
| NMC | Nursing and Midwifery Council |
| NSA | Non-substantial amendment |
| NRES | National Research Ethics service |
| SA | Substantial amendment |
| R and D | Research and development |
| REC | Research Ethics Committee |
| R and I panel | Research and innovation panel |
| RMG | Research governance manager |
11.2 Appendix B research and innovation panel composition
11.2.1 Membership
11.2.1.1 Quoracy, 3 members required
- Deputy medical director (co-chair).
- Director of research and innovation (co-chair).
- Deputy director of research and innovation.
- Clinical director for research (or nominated deputy).
- Consultant nutritionist.
- Chief pharmacist (or nominated deputy).
- Clinical director psychological therapies research.
- Research and innovation manager (once in post).
- Clinical effectiveness lead, nursing and quality.
- Senior finance business partner.
- Research governance manager.
- Clinical care group directors (or nominated deputy).
11.2.1.2 Other membership
- Deputy clinical director for research.
- Post-doctoral research associates.
- Clinical research fellows.
- Clinical research nurses.
- Clinical studies officers.
- Research support officers.
- Research assistants.
- Communications and engagement officer.
- Research Governance team.
- Administrative team.
- Any other members of the Grounded Research team.
- Research active clinicians, nurses or allied health professionals with special interest in research and innovation.
- Patient research ambassador (PRA).
11.2.1 Ad hoc attendance
- For quoracy, 3 members of “11.2.1.1 Quoracy, 3 members required”
- Representatives from other trust services will be invited in accordance with agenda items.
- Upon invitation any member of trust staff with a special interest can attend upon invitation from the chair.
11.3 Appendix C UK framework for health and social care research
The UK Framework for Health and Social Care Research mandate states that the following core principles apply to all health and social care research.
11.3.1 Principle 1 safety
The safety and well-being of the individual prevail over the interests of science and society.
11.3.2 Principle 2 competence
All the people involved in managing and conducting a research project are qualified by education, training and experience, or otherwise competent under the supervision of a suitably qualified person, to perform their tasks.
11.3.3 Principle 3 scientific and ethical conduct
Research projects are scientifically sound and guided by ethical principles in all their aspects.
11.3.4 Principle 4 patient, service user and public involvement
Patients, service users and the public are involved in the design, management, conduct and dissemination of research, unless otherwise justified.
11.3.5 Principle 5 integrity, quality and transparency
Research is designed, reviewed, managed and undertaken in a way that ensures integrity, quality and transparency.
11.3.6 Principle 6 protocol
The design and procedure of the research are clearly described and justified in a research proposal or protocol, where applicable conforming to a standard template and, or specified contents.
11.3.7 Principle 7 legality
The researchers and sponsor familiarise themselves with relevant legislation and guidance in respect of managing and conducting the research.
11.3.8 Principle 8 benefits and risks
Before the research project is started, any anticipated benefit for the individual participant and other present and future recipients of the health or social care in question is weighed against the foreseeable risks and inconveniences once they have been mitigated.
11.3.9 Principle 9 approval
A research project is started only if a research ethics committee and any other relevant approval body have favourably reviewed the research proposal or protocol and related information, where their review is expected or required.
11.3.10 Principle 10 information about the research
In order to avoid waste, information about research projects (other than those for educational purposes) is made publicly available before they start (unless a deferral is agreed by or on behalf of the research ethics committee).
11.3.11 Principle 11 accessible findings
Other than research for educational purposes and early phase trials, the findings, whether positive or negative, are made accessible, with adequate consent and privacy safeguards, in a timely manner after they have finished, in compliance with any applicable regulatory standards, for example, legal requirements or expectations of regulators. In addition, where appropriate, information about the findings of the research is available, in a suitable format and timely manner, to those who took part in it, unless otherwise justified.
11.3.12 Principle 12 choice
Research participants are afforded respect and autonomy, taking account of their capacity to understand. Where there is a difference between the research and the standard practice that they might otherwise experience, research participants are given information to understand the distinction and make a choice, unless a research ethics committee agrees otherwise. Where participants’ explicit consent is sought, it is voluntary and informed. Where consent is refused or withdrawn, this is done without reprisal.
11.4 Appendix D confirmation of NHS trust capacity and capability
Confirmation of NHS trust Capacity and Capability is the local feasibility procedure undertaken by an NHS Organisation to assess and confirm whether the organisation has the resources, policies and service users required to successfully deliver the research study to time and target.
The NHS research and development department involved with the research project will conduct a formal assessment to establish feasibility of the research study by the following.
11.4.1 Assessing
Assessing if the trust has the capacity and capability to participate in the study by reviewing:
- minimum document set received
- principal investigator or local collaborator required
- required population to meet the recruitment target
- staff available to deliver the study
- site selection visit
11.4.2 Arranging
Put practical arrangements into place to provide the capacity and capability to deliver the study successfully:
- agreement of local information document
- agreement of schedule of events (SoE) or schedule of events cost attribution template (SoECAT)
- study specific training
- confirmation from services involved
- confirmation from outsourced services
- agreement of financial arrangements
- letters of access or honorary contracts
11.4.3 Confirming
Confirm that the trust has the capacity and capability to deliver the study to time and target, through mutual confirmation of the Statement of activities for non-commercial studies or sign-off of a formal agreement between the sponsor and the trust.
- Health Research Authority approval received.
- Fully executed contract, agreement and, or organisation information document.
- Confirmation email of capacity and capability.
- Green light from sponsor.
11.5 Appendix E research study approvals process
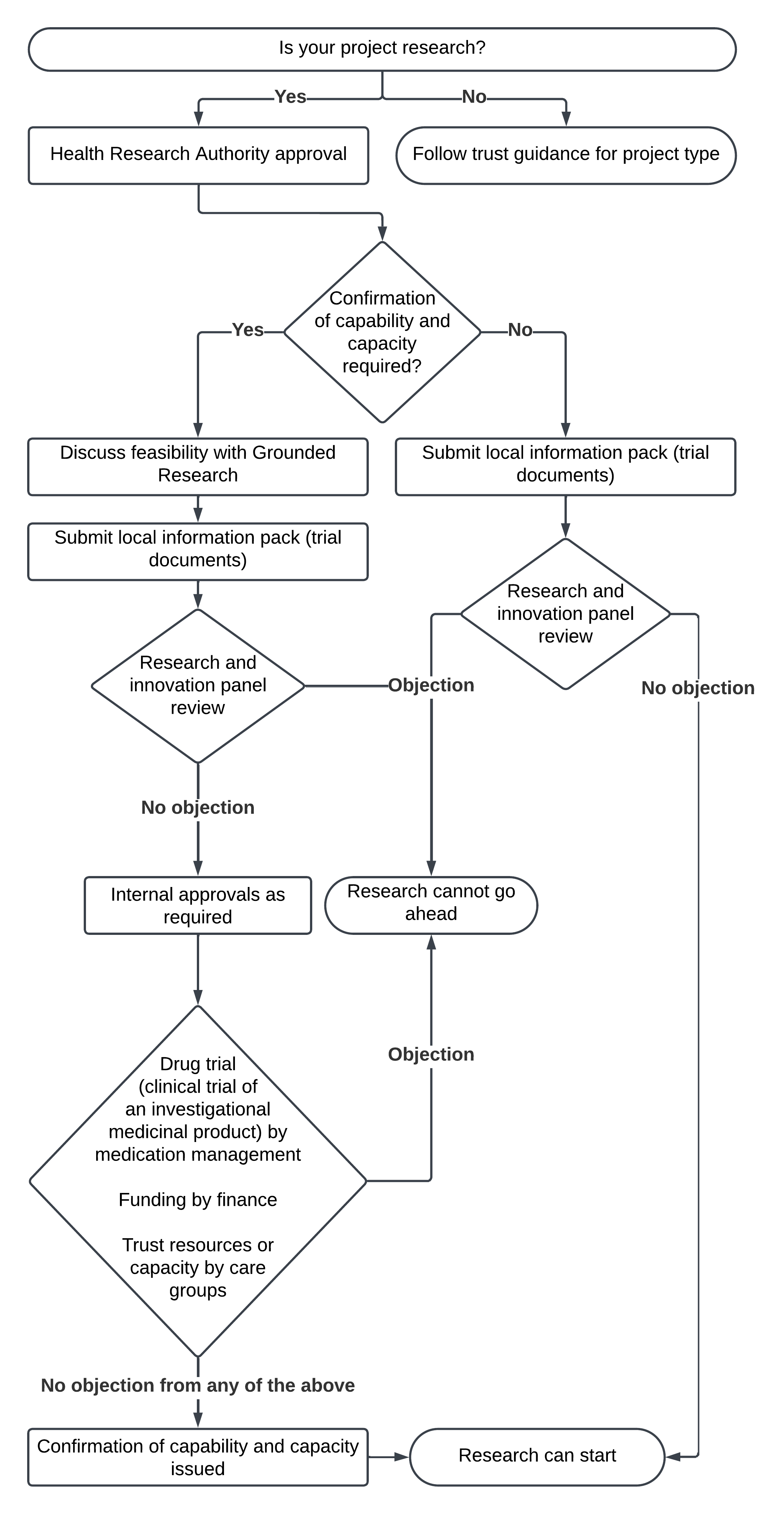
- Is your project research?
- No, follow trust guidance for project type.
- Yes, Health Research Authority approval, does the Health Research Authority deem that confirmation of capability and capacity is required?
- Yes, discuss feasibility with Grounded Research then submit local information pack (trial documents), the research and innovation panel then reviews:
- if there is an objection research cannot go ahead
- if there is no objection, internal approvals are required from drug trial (clinical trial of an investigational medicinal product) by medication management,
funding by finance
and trust resources or capacity by care groups:
- if there is an objection research cannot go ahead
- if there are no objections from any of the above, confirmation of capability and capacity is issue and research can start
- No, submit local information pack (trial documents), the research and innovation panel then reviews:
- if there is an objection research cannot go ahead
- if there is no objection research can start
- Yes, discuss feasibility with Grounded Research then submit local information pack (trial documents), the research and innovation panel then reviews:
Document control
- Version: 8.1.
- Unique reference number: 335.
- Approved by: research and innovation clinical leadership executive group.
- Date approved: 14 January 2025.
- Name of originator or author: research governance manager.
- Name of responsible individual: medical director.
- Date issued: 25 October 2025.
- Review date: 31 January 2028.
- Target audience: everyone involved in the trust.
Page last reviewed: October 28, 2025
Next review due: October 28, 2026
Problem with this page?
Please tell us about any problems you have found with this web page.