Contents
1 Introduction
RDaSH are committed to ensuring that a consistent approach is taken to inform service delivery decisions, business cases, savings plans and any other business plans, including a robust evaluation for their impact on healthcare quality.
Healthcare systems are increasingly complex with a growing number of interdependencies, some of which sit outside the traditional range of NHS funded services. The gap between healthcare demand and available capacity has contributed to a significant challenge for the NHS and provider organisations are required to make increasingly difficult decisions about the viability and range of local services.
This has become more significant following the two years of gold command in RDaSH and the acknowledgement nationally that waiting lists for services require fresh approaches.
The fresh approach in this policy can be described as a collaborative approach. The QSIA approval process, recommendations and discussions will be a partnership between operational services the nursing and quality directorate, medical director’s office, and the Finance and Performance team. QSIA methodology forms part of the cycle of business in the trust.
Significant changes are required to address the recognised variance between healthcare demand and capacity, to improve the health of our local population through better prevention of health need and to enable services to work in an effective and efficient way.
Achieving this may require changes in local service pathways. Service changes require robust planning and implementation to ensure that the potential for unintended harmful impacts on patient safety, clinical effectiveness or patient experience are proactively prevented, and mitigated.
This policy describes how the offices of the medical director, chief operating officer, director of finance, director of people and organisation development and the chief nurse will work collaboratively to achieve this.
2 Purpose
The purpose of this policy is to set out the principles, process, and format to be followed to ensure that changes, initiated as a consequence of service delivery or savings decisions made by the trust, are fully assessed for their impact.
Impact assessment must consider the positive impact expected on healthcare quality, ensure that any known or expected negative impact on patient safety and quality is robustly assessed and understood and ensure that any potential unintended negative consequences are identified and mitigated.
The assessments must also consider the short, medium, and long-term impact of any changes being proposed. The intention of a change may have positive long-term impacts however implementing the change may produce negative short-term impacts which need to be considered as part of the QSIA process.
This is the process for gaining assurance of the quality and safety impacts of service changes, cost savings plans, and other change projects that may affect the delivery of operational services and gaining assurance of the risk management and impact mitigation strategies used to control negative impacts.
The impact is tested through an evidence-supported narrative and rated using a scale from negative to positive to allow for risks and benefits to be quantified and described.
3 Scope
This policy applies to all services, teams and staff members that lead or seek assurance about service developments, and re-design programmes. It includes financial savings programmes and any other developments that result in healthcare service change.
The policy will provide guidance for completing the QSIA checklist and the full QSIA tracker and will outline the main responsibilities of key post holders involved in the process.
4 Quality and safety impact assessment process
Section 4.2.1 shows a depiction of the process that will be followed when approving and reviewing QSIA checklists and full QSIAs.
All new projects, efficiency and cost improvement plans, business cases, service redesign, efficiency strategies and any other plans for change initiated by the trust will be required to complete a QSIA checklist (appendix A) to outline any potential impacts. To do this effectively, the right information is needed in order to understand the potential risks to quality and plans must be put in place to ensure action is taken before quality deteriorates.
Process in brief: the QSIA checklist template will require a brief description of the project, outlining the purpose, the problem and the proposal. It includes the key actions, how the benefit will be realised, and if there are any workforce impacts. This will ensure that the QSIA review panel have the appropriate information required to fully understand.
The QSIA checklist will be completed in the planning stage of the project development. This assessment will form part of the overall approval process for the change project, and approval of the QSIA should not be considered approval to commence the project, other approval processed may be required dependent the application of other trust policies.
If there is a negative impact on quality or safety that is identified on the initial checklist, then a full QSIA will be required. The purpose of the full QSIA is to provide specific detail about what risks exist as a result of the change, and how the project team will mitigate against the risks.
4.1 Step 1 checklist
The project lead will complete the QSIA checklist (appendix A), this details whether there is potential quality and safety impact of the proposal.
4.1.1 Where there are no potential impact on quality and or safety
The project lead will submit the checklist to the QSIA panel for review.
The QSIA panel will review the information presented and will judge if they are assured by the information and assessment presented. If the QSIA panel are assured the assessment will be supported and the project can be initiated or progress to the next stage of approval dependent on other policies that might apply.
It is best practice for the project lead or team to continue to assess if quality or safety is being impacted during the execution of the project, even if the initial assessment did not identify and impacts.
4.1.2 Where there are potential impacts on quality and or safety
The project lead will complete step 2, full assessment detailed in 4.2 of this policy.
4.2 Step 2, full assessment
4.2.1 Where there are potential impacts on quality and or safety
The project or clinical lead will complete the full QSIA (appendix B), this will detail the specific impact and risks associated with that impact, how the risk will be mitigated, how the impact will be monitored and what will trigger further mitigation or escalation.
The project lead will submit the checklist and the full QSIA to the QSIA panel for review.
The QSIA panel will review the information presented and will judge if they are assured by the information and assessment presented. If the QSIA panel are assured the project can be initiated or progress to the next stage of approval dependent on other policies that might apply.
The QSIA panel may request the project to resubmit the full QSIA routinely to maintain assurance that risks and being appropriately mitigated.
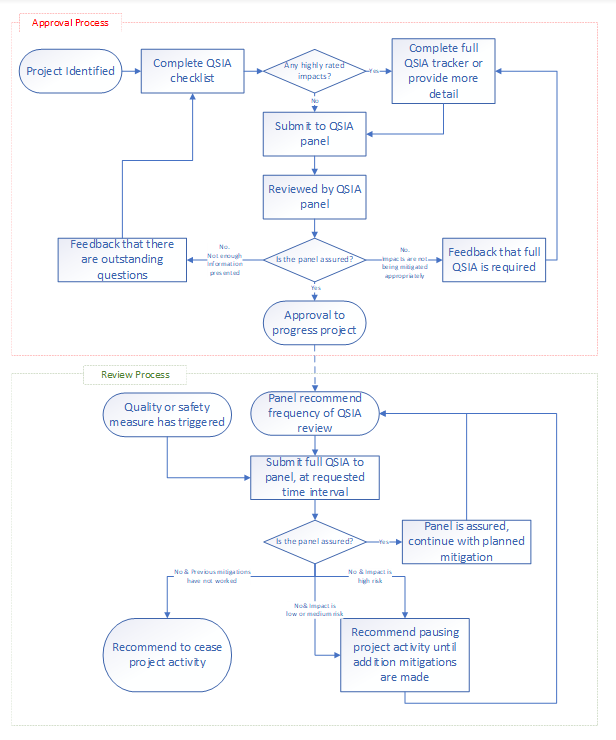
4.2.2 Description of process
4.2.2.1 Quality and safety impact assessment approval process
- The process starts when a project is identified.
- Project lead completes the QSIA checklist.
- Are there any highly rated impacted identified when completing the QSIA checklist?
- yes, the project lead completes the full QSIA tracker, or provides more detail to the QSIA checklist
- Project lead submits the QSIA checklist and, or full QSIA tracker to the QSIA panel.
- The QSIA panel will meet to review the submitted QSIA checklists and full QSIA trackers.
- Is the panel assured?
- yes, the panel approves the project to progress and may recommend to the project lead how frequently the QSIA should be resubmitted and reviewed by the panel
- no, the panel will feed back that a full QSIA tracker is required before approval is granted
4.2.2.2 Quality and safety impact assessment review process
- At step 6 of the QSIA approval process the panel will have recommended how frequently the QSIA should be resubmitted and reviewed by the panel.
Or:
- A quality or safety measure has been triggered.
- The project lead submits a full QSIA tracker to the QSIA Panel at the requested time interval.
- Is the QSIA panel assured?
- Yes, the project lead should continue with the project with the planned mitigating actions.
- No and previous mitigations have not worked? The QSIA panel will recommend ceasing the project activity.
- No and impact is low or medium risk? The QSIA panel will recommend pausing the project activity until additional mitigating actions are made, proceed back to step 2.
- No and impact is high risk? The QSIA panel will recommend pausing the project activity until additional mitigating actions are made, proceed back to step 2 of the QSIA review process.
5 Responsibilities, accountabilities and duties
The roles and responsibilities for quality and safety impact assessments are set out below:
5.1 The chief nurse
The chief nurse and their team, will be responsible for ensuring that quality and safety impact assessments are effectively considered and undertaken as part of discussions and decisions about business cases relating to financial recovery, service transformation or other business plans affecting service delivery change.
The chief nurse will be responsible for seeking assurance that RDaSH and any sub contractors have robust systems in place to undertake QSIAs for all new projects, efficiency and cost improvement plans, business cases, service redesign, efficiency strategies and any other plans for change initiated by the trust.
Chief nurse will be responsible for ensuring that there is a multi-disciplined panel who will review and approve the QSIA checklists and full QSIAs.
5.2 The quality and safety impact assessment panel
The QSIA panel should be made up of directors or delegated deputies from:
- nursing and quality
- medical director’s office
- therapies directorate
- finance and performance directorate
- director of people and organisation development
- operational services
This will ensure that there is a multi-disciplined review of all QSIAs and full assessments and that the process is well led from floor to board.
The panel will meet frequently to review the submitted QSIA.
It will be the responsibility of the whole panel to make a judgement of assurance based on the information and impact assessment being presented. The panel will consider questions such as “is the panel assured that the assessment is a true reflection of the project actions described?”, “are the mitigations suitable for the scale of risk?”, and “will the project have any unforeseen impacts on other services beyond the area presenting the assessment?”
The QSIA panel will agree on a frequency that full QSIAs are to be resubmitted to the panel for monitoring.
When monitoring a full QSIA the panel should review the assessment and seek assurance that quality and safety metrics are being monitored by the project lead and team, and that these metrics have not deteriorated as a result of the project.
5.2.1 Patient safety focus
If quality and safety data is showing outside tolerance levels (on a dashboard for example) or if the panel is not assured that appropriate mitigating actions are being taken, there are three options that can be taken:
- recommend that the project be stopped immediately
- recommend that the project be paused until further mitigations are put in place
- recommend that further mitigations are put in place but without stopped or slowing the project deliver
5.2.2 Cumulative impact assessment
The panel will retrospectively review the impact assessments that have been submitted within the past six months to consider if there are any cumulative or aggregated impacts from the changes made in that period.
5.3 The trust board, executive directors and non-executive board members
The executive directors and non-board members are responsible for ensuring that financial and operational projects (for example, business cases, service re-design, cost saving strategies and other business plans) have been evaluated for their impact on quality and have assured themselves that minimum standards will not be compromised.
The chief operating officer has responsibility for the quality assurance of service delivery decisions across the organisation, as part of the RDaSH board.
5.4 Trust operating model
The QSIA panel will provide an out-brief for the quality and safety group. This is a sub-group to the clinical leadership executive (CLE).
The finance group also receives the same brief analysing it with a different lens, reporting up to CLE.
The CLE then reports into The trust’s board of directors.
5.5 Quality Committee
Quality Committee will be responsible for assuring the board and others that the impact on quality and patient safety is monitored appropriately throughout the introduction of service change, via highlight reports and the risk log in order to ensure that emerging unintended impact is identified and mitigated or escalated to the board.
Quality committee will receive a report twice a year on the activity and effectiveness of the QSIA process as part of the clinical governance process.
5.6 All employees of RDaSH
Any individual with lead responsibility for completing or preparing a policy, strategy, business case, service re-design, or other plan can be the responsible author for a quality safety impact assessment (QSIA).
The QSIA author is responsible for ensuring that the checklist, and where applicable the full QSIA, is completed, recorded, approved, and reviewed in accordance with this policy and any related operational procedure guidance.
6 Practical application of policy
6.1 When should a quality and safety impact assessment be undertaken?
- A QSIA must be initiated as part of the development and proposal stage of all, service changes, business cases, savings plans, and other change projects, that have a direct or indirect impact on patient care.
- QSIAs will be initiated in order that necessary mitigation can be agreed which should become an essential component of project implementation plans.
- QSIA approval must be in place before a project commences implementation, ideally. The QSIA approval process is outlined in section 4.2.1.
- The QSIA checklist should be reviewed and updated as part of other ongoing evaluation of a project during implementation.
- Where the components of a project change and delivery is modified due to the emergence of new evidence or performance data, an updated QSIA checklist should be submitted for review by the panel.
6.2 Why should a quality and safety impact assessment be undertaken?
Completion of the QSIA is a continuous and dynamic process to help decision makers fully think through and understand the consequences and potential impact of financial, quality improvement and operational projects, with a focus on patient safety.
They support evidence of fair and proportionate reasoning and a consistent process for making decisions about local healthcare services.
They provide assurance that actual or potential risks to patients have been sufficiently considered and mitigated, particularly for decisions that may be considered contentious.
A QSIA will help the trust avoid false assurance regarding any change project that may have been underpinned by anecdotal or subjective opinion.
Undertaking a QSIA requires close collaboration between project leads (care groups or corporate teams) and clinicians and this supports a framework for engagement and strengthens the rationale for the implementation of a project.
6.3 How often should a quality and safety impact assessment be undertaken?
QSIA checklists should be reviewed on a regular basis by the project lead. The frequency will depend on the scale and complexity of the changes and will be at the project lead’s discretion at a minimum of quarterly. The frequency should be proportionate to the level of risk identified and the expected response time of the project outcome.
Where a full QSIA is required the frequency of a formal review and monitoring will be recommended by the QSIA panel. It is suggested when there is a full QSIA that the identified quality and safety metrics are monitored at least monthly.
When the full QSIA is submitted to the panel for monitoring any new or additional quality and safety impacts should be assessed and documented prior to submission to the panel.
7 References
- National quality board (2013) How to: quality impact assess provider cost improvement plans.
- NHS providers (2015) Good practice in quality impact assessment. Foundation trust network.
8 Appendices
8.1 Appendix A The quality and safety impact assessment checklist
8.2 Appendix B The quality and safety impact assessment full assessment
8.3 Appendix C Terms of reference for a quality and safety impact assessment panel
8.3.1 Terms of reference
8.3.1.1 Purpose
The purpose of the panel is to provide leadership for the effective management of quality and safety during any proposed changes affecting or impacting on clinical services. This will be achieved by ensuring there is a formal review of all quality and safety impact assessments (QSIA) in line with the QSIA policy, and that the process of change is well led from floor to board.
The panel will receive and review QSIAs from project leads. Information will be presented to the panel who will provide a judgement regarding the quality and safety of patients and the actions being taken by the project team to mitigate negative impacts. This will lead to the panel either supporting the assessment that is being presented or recommending further actions are taken to reduce or remove the impact of risks to quality or safety.
The panel will consider the positive impact expected on healthcare quality, ensuring that any known or expected negative impacts on patient safety and quality have been assessed and understood by the project lead, and ensure that any potential unintended negative consequences are identified and robustly mitigated.
This formal review will support the effective delivery of the savings programme, business case development process, and proposed service improvement changes to services in RDaSH.
8.3.1.2 Responsible to
- Trust board of directors via quality and safety group and quality committee, and the clinical leadership executive subgroup, the finance group.
8.3.1.3 Objectives
- Ensure there is a multi-disciplined, cross-organisational evaluation of projects that may impact on operational services.
- To review and evaluate QSIA checklists for all projects which are submitted by project leads.
- To establish if assurance has been provided that there are no impacts on quality or safety.
- Where assurance has not been provided through the use of the QSIA checklist, the panel will ensure that a full QSIA tracker is submitted. This then provides additional information about how risks to quality and/or safety are going to be managed.
- To review and evaluate QSIA trackers for all projects where risks to quality and/or safety have been identified.
- Provide project leads with feedback that their assessment of quality and safety impacts is supported by the panel and the project may progress to it next approval process as appropriate.
- Gain assurance that quality and safety risks are being managed through requesting project leads to resubmit QSIA trackers periodically.
- Provide a point of escalation for project leads when quality and/or safety measures are triggered.
8.3.1.4 Membership
The QSIA panel should be made up of directors or delegated deputies from:
- Nursing and quality directorate (Chair)
- Medical director’s office
- Therapies directorate
- Finance and performance directorate
- People and organisation development directorate representative
- Equality, Diversity, and Inclusion team
- One operational services representative, chief operating officer (or deputy), care group director, director of nursing (care groups), or medical director of the care group
8.3.1.5 Frequency
Meetings will be held as frequently as required. This will be dependent on the volume of assessments submitted.
8.3.1.6 Quorum
Not a decision-making group therefore quoracy is not a factor.
8.3.1.7 Support arrangements
Administrative support to be provided by the nursing and quality directorate.
Project management to be provided by the Cost Savings team and finance.
8.3.1.8 Governance, rules and behaviours
- QSIA checklists and assessments will be shared with the panel 5 working days ahead of the meeting and it is expected that members will have reviewed the information before the meeting.
- If there is the requirement for rapid assessment, a QSIA checklist may be circulated via email to the panel for review and comments will be collected by email reply. At the next available meeting there will be a summary record made in the meeting notes to formalise the outcome.
- Declaration and conflicts of interest in relation to the project assessments being reviewed at the meeting must be made at the start of the meeting.
- Each representative will review the submitted information and provide a judgement of assurance from the perspective of their professional area.
- The chair has overriding authority for concluding the judgement of the panel.
- All members are required to attend, a delegate with the appropriate authority to make appropriate challenge and recommendations must be identified for any known absences.
- Meetings will start and end on time.
- Meeting will be held either using video-conferencing software, or face-to-face. Under exceptional circumstances (for example, where rapid decision-making is required) assessments may be reviewed via email and the chair will review responses to make the decision to approve.
- Notes of the meeting will be made, which will document the title of the QSIA being presented, the judgement the panel made, and what quality metrics are being monitoring (were appropriate).
- The chair has authority to cancel meeting.
8.3.1.9 Standing agenda items
- Declarations and conflicts of interest.
- Review of submitted QSIA checklists.
- Review of new full QSIA trackers submitted.
- Review of re-submitted QSIA trackers.
- Horizon scan, future project or potential changes.
To access the equality impact assessment for this policy, please email rdash.equalityanddiversity@nhs.net to request the document.
Document control
- Version: 2.
- Unique reference number: 1008.
- Approved by: Quality and safety CLE group.
- Date approved: 14 May 2024.
- Name of originator or author: Senior programme manager.
- Name of responsible individual: Chief nurse.
- Date Issued: 6 August 2024.
- Review date: 31 August 2027.
- Target audience: Trust wide.
Page last reviewed: January 21, 2025
Next review due: January 21, 2026
Problem with this page?
Please tell us about any problems you have found with this web page.
Report a problem