Contents
1 Aim
This SOP is aimed at all researchers who do not already have an appropriate contractual relationship in place and want to undertake research activities in the trust. It sets out the trust procedure for applying for an honorary research contract (HRC) or letter of access (LoA). It also covers honorary collaborative contracts (HCC) issued to acknowledge a collaborative relationship with a researcher.
The SOP is applicable to Grounded Research staff involved in the assessment and issuing of RPs, HRCs, LoA and HCCs, setting out the process for validation and review of research passports and for issuing and monitoring HRCs, LoAs and HCCs.
2 Scope
This SOP has been developed with the support of the RDaSH The People Experience team (HR) department and is in accordance with the national NIHR ‘Research in the NHS: HR Good Practice Resource pack’.
The HCC process has been agreed with the RDaSH Executive Management team.
The RP scheme was developed by DHSC and the United Kingdom Clinical Research Collaboration (UKCRC).
3 Link to overarching policy, and or procedure
4 Procedure or implementation
4.1 Streamlined the people experience team arrangements
Also refer to the HR good practice resource pack for HR and contractual arrangements. These documents are hosted on the IRAS website (opens in new window).
The Department of Health and Social Care (DHSC) Framework for Health and Social Care Research requires that all NHS trusts ensure that individuals undertaking research that involves NHS staff or patients, their organs, tissue or data must have either a substantive contract, HRC or a LoA with the NHS.
Where access to an NHS organisation is not covered by a substantive employment contract or an honorary clinical contract with one NHS organisation the RP is in place to streamline the HR arrangements to obtain access.
The HR Good Practice Resource Pack was reviewed and updated in light of the Data Protection Act 2018 (DPA 2018) and the General Data Protection Regulation (GDPR) which came into force in the UK on 25 May 2018. The data requested in the research passport application form has been minimised following discussion with data protection and information governance officers and human resource experts.
There are two pathways in the RP scheme which are managed depending on the employing organisation, where researchers have a substantive employment contract or HCC with an NHS organisation the NHS to NHS (hosted on IRAS website) LoA can be issued to allow research access to another NHS trust. For staff not employed by an NHS organisation the RP process enables HEI and other employers to share pre-engagement information about their researcher with NHS organisations hosting the researcher’s activity (see appendix B).
4.2 NHS to NHS arrangements
Where NHS staff want to undertake research within the NHS but outside of their employing trust the NHS to NHS Proforma streamlines the process to obtain access to other NHS organisations. The process allows trusts to share pre-engagement checks to prevent the duplication of checks by multiple trusts thereby allowing requests to be processed quickly. Staff who are conducting research as part of their clinical honorary contract with RDaSH do not require an HRC or LoA.
4.2.1 To obtain a NHS to NHS letter of access
To obtain a NHS to NHS letter of access to undertake research activities in another trust, RDaSH substantive employees should download and complete the NHS to NHS confirmation of pre-engagement checks proforma.
The completed form is sent, along with a current CV, to their head of department or line manager who should sign the form as the employer’s representative and return it to the applicant.
The applicant should then submit the completed form to all NHS trusts in which an NHS to NHS LoA is required.
4.2.2 On receipt of an NHS to NHS letter of access application from another trust
Grounded Research, on behalf of the trust, will accept the NHS to NHS Proforma as confirmation of pre-engagement checks from the researcher’s substantive employer as evidence that the appropriate clearances are in place and inform the researcher’s substantive employer of her or his activities in their organisations by issuing the NHS to NHS LoA.
Grounded Research governance staff will check that the NHS to NHS Proforma has been completed correctly and has been dated. If the form is complete, and any required supporting documents have been provided, the research governance manager will issue an NHS to NHS LoA. A copy of the LoA and NHS Proforma will be sent to the person requesting access and the signee of the NHS Proforma.
The proforma and a copy of the LoA will be filed in the HRC or LoA folder and details (including contact details, study title and expiry date) will be recorded on the HRC or LoA tracker.
4.3 Research passport (HEI)
The RP system is used for university employed researchers and where students are undertaking research as part of a Masters, PhD, or other qualification where the course is not subject to a healthcare placement agreement with clinical supervision. For students the HEI would need to ensure that the student admissions are able to complete the sections of the RP normally completed by HR for employed staff.
Students conducting research as part of their healthcare placements should not be issued with HRC or LoA.
Depending on the activities the researcher will be undertaking either a HRC or a LoA will be issued. The research passport algorithm sets out the type of access and checks that are required for different levels of research activity (see appendix C).
4.3.1 To obtain a research passport
To obtain a research passport to undertake research activities in a NHS organisation the non-NHS employee should download and complete the research passport form from the IRAS website.
The completed form must be validated by the substantive employer or registry at place of study for students. The line manager or academic supervisor must sign the form to confirm suitability of the researcher (section 4) and the HR department or registry must complete details of pre-engagement checks (section 5).
The applicant should then submit the completed form to the lead NHS organisation for validation, including original copies of their CV, DBS disclosure certificate, and evidence of occupational health clearance. The lead NHS organisation reviews the RP and may decide that additional checks are required. Once they are satisfied that sufficient checks have been completed the RP is validated and a HR or LoA is issued.
The validated RP can then be sent to all NHS trusts in which an HRC or LoA is required. It is not required to submit copies of supporting documents such as the DBS certificate, occupational health clearance forms and evidence of qualifications to the NHS trusts. Detailed instructions for completion of the RP are available on the IRAS website.
4.3.2 On receipt of a validated research passport from a HEI or NIHR partner organisation
Grounded Research, on behalf of the trust, will accept the RP as confirmation of pre-engagement checks and as evidence that the appropriate clearances are in place and inform the researcher’s substantive employer of her or his activities in their organisations by issuing the HRC or LoA.
Grounded Research will check that the RP has been completed correctly, validated by the lead NHS Organisation and has been signed and dated. If the form is complete, and any required supporting documents have been provided, the research governance manager will issue a HRC or LoA. A copy of the HRC or LoA will be sent to the applicant and their line manager and HR contact as listed on the RP.
The RP and a copy of the HRC or LoA will be filed in the HRC or LoA folder and details (including contact details, study title and expiry date) will be recorded on the HRC or LoA tracker.
4.3.3 Further information
Documents and frequently asked questions:
- FAQs HR and contractual arrangements (opens in new window)
- FAQs managing and monitoring the RP system (opens in new window)
- documents, background information documents
- documents honorary research contracts principles and legal requirements (opens in new window)
4.4 Honorary collaborative contract
In some circumstances Grounded Research requires an agreement to facilitate a collaborative research relationship outside the remit of the RP scheme; for example this includes work on joint grant award bids or collaborative work for protocol development. In these circumstances an honorary collaborative contract (HCC) can be issued.
The HCC form will be approved by the research panel and then signed by the research collaborator. The partially signed contract is sent to the executive medical director or director of research for signature on behalf of the trust. They are responsible for updating the Executive Management team (EMT) on a regular basis as to which contracts have been signed off.
In addition to an HCC consideration must be given as to whether any other form of formal agreement or contract is required, including but not limited to; a collaboration agreement, memorandum of understanding, or service level agreement. Where required advice should be sought from the contracts department to determine the correct route to take.
In the event that there is a potential for new intellectual property the intellectual property exploitation policy must be considered.
4.5 Managing and monitoring
Also refer to the FAQs managing and monitoring research passports, on the IRAS website.
No HRC or LoA may commence until the principal investigator for the research project at RDaSH has received confirmation that the trust has the capability and capacity to undertake the research project. During the assessment of capability and capacity, the research governance manager is responsible for checking that HRC or LoA arrangements are in place or have been initiated where required.
4.5.1 On receipt of a research passport or NHS to NHS proforma
On receipt of a research passport or NHS to NHS Proforma the research governance manager will review and confirm that the form has been fully completed, signed and any supporting documents have been provided. If the application is complete the appropriate access letter will be issued.
The documents and a copy of the HRC or LoA letter will be filed in the HRC or LoA folder and details (including contact details, study title and expiry date) will be recorded on the HRC or LoA tracker.
4.5.2 Concerns and queries
In cases where the research governance manager has reason to think that additional checks might be required, and or there is any doubt with regard to the substantive employer’s compliance with the scheme, or there are other queries, advice must be sought from the HR department (see appendix A).
For the NHS to NHS proforma where the employer’s compliance cannot be ascertained host organisations can issue the letter of joint arrangements alongside the NHS to NHS LoA. Further details can be obtained from the “FAQs, HR and Contractual Arrangements” document point 6, page 5, available on the IRAS website.
For research passports additional checks can be requested by the Lead NHS Organisation validating the RP and should be processed by the HR department of the lead NHS organisation. Where additional checks are carried out details must be recorded in section 7 of the RP.
4.5.3 Amendments and extensions
Grounded Research is responsible for monitoring active HRCs, LoAs and HCCs, all of which must be given a start and end date corresponding with the expected duration of the research project or collaboration for which access has been issued. Where a researcher is on a fixed term contract with an end date before the end of the project access can only be given until the contract end date.
For RP applications where the end date of a project, or fixed term contract, is extended the researcher can apply for an extension to their access period using the passport appendix (list of amendments to the RP). This appendix can also be used to obtain access to work at additional sites, and to add additional research projects at a site where study specific access has already been given.
A RP is only valid for a maximum of three years from the date validated by the employing organisation. If access is required after that date a new validated RP will be required.
For NHS-to-NHS applications a new proforma with the new end date will be required. It is not necessary to submit a new proforma to make changes or extend access to additional studies, a letter or email with the details is sufficient.
It is the responsibility of the researcher to notify Grounded Research of any changes to the information provided to obtain an HRC or LoA. This includes but is not limited to change to:
- substantive employer
- criminal Record
- health condition or disability that may affect the research role
- end date of a fixed term contract
- end date of required access
- addition of studies to a research passport
- study role(s) listed on a research passport
- contact details
In the event that any of the conditions and expectations as set out in a HRC or LoA is not adhered to access may be terminated early in accordance with the clauses in the contract. Amendments to an existing RP will be reviewed by the research governance manager and recorded on the HRC or LoA tracker.
4.5.4 Monitoring
Grounded Research will maintain a tracker detailing all active HRCs, LoAs and HCCs. A check will be carried out at least once a month to identify HRCs, LoAs and HCCs due to expire the following month. A courtesy email will be sent as a reminder that, where access is still required after the end date, an extension is required. If an extension is not requested access will cease on the date given on the HRC, LoA or HCC.
HRCs, LoAs and HCCs may be subject to random checks carried out by Grounded Research within the lifetime of the project or collaboration.
5 Summary
The research passport system provides a mechanism for higher education (HE) employers to share pre-engagement information about a researcher with relevant NHS organisations in which that researcher will be conducting their research activity. The research passport system provides:
- clear guidance on the relevant checks required
- a robust process for HE employers to document and evidence the checks which have been undertaken
- clear principles that enable NHS organisations to have a record of and rely on those checks for the duration of the Research Passport
In RDaSH responsibility for managing and monitoring the RP system is delegated by HR to Grounded Research.
6 References
- Health Research Authority. (2018) UK Policy Framework for Health and Social Care (accessed 14 April 2022) (opens in new window).
- HR Good Practice Resource Pack (accessed 14 April 2022) (opens in new window).
- The UK Clinical Research Collaboration (UKCRC) (accessed 14 April 2022) (opens in new window).
7 Appendices
7.1 Appendix A Flowchart
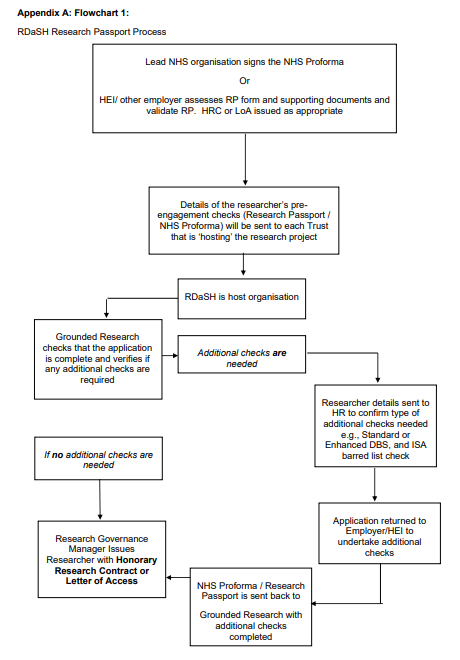
- Lead NHS organisation signs the NHS Proforma Or HEI or other employer assesses RP form and supporting documents and validate RP. HRC or LoA issued as appropriate.
- Details of the researcher’s pre-engagement checks (research passport or NHS Proforma) will be sent to each trust that is hosting the research project.
- RDaSH is host organisation.
- Grounded Research checks that the application is complete and verifies if any additional checks are required.
- Additional checks are needed.
- Researcher details sent to HR to confirm type of additional checks needed for example, standard or enhanced DBS, and ISA barred list check.
- Application returned to employer or HEI to undertake additional checks.
- NHS Proforma or research passport is sent back to Grounded Research with additional checks completed.
- Research governance manager issues researcher with honorary research contract or letter of access (if no additional checks are
needed).
7.2 Appendix B Summary of forms of contractual arrangement available for individuals undertaking research in the NHS
7.3 Appendix C Research passport algorithm
7.3.1 Researcher is a health care professional providing health care to an adult and, or child
7.3.1.1 Criminal record check necessary?
- Yes, if done once this is regulated activity (new definition). Requires enhanced DBS and appropriate barred list check.
7.3.1.2 Occupational health clearance necessary?
- Yes, if there is direct contact.
7.3.1.3 LOA or HRC?
- HRC.
7.3.2 Researcher provides health care to an adult and, or child under the direction or supervision of a health care professional
7.3.2.1 Criminal record check necessary?
- Yes, if done once this is regulated activity (new definition). Requires enhanced DBS and appropriate barred list check
7.3.2.2 Occupational health clearance necessary?
- Yes, if there is direct contact.
7.3.2.3 LOA or HRC?
- HRC.
7.3.3 Researcher provides personal care to an adult or child or researcher is a social care worker providing social work which is required in connection with any health care or social services to an adults who is a client or potential client
7.3.3.1 Criminal record check necessary?
- Yes, if done once this is regulated activity (new definition). Requires enhanced DBS and appropriate barred list check
7.3.3.2 Occupational health clearance necessary?
- Yes, if there is direct contact.
7.3.3.3 LOA or HRC?
- HRC.
7.3.4 Researcher undertakes the following activities unsupervised: teach, train, instruct, care for or supervise children, or provide advice or guidance on wellbeing, or drive a vehicle only for children with likely direct bearing on the quality of care
7.3.4.1 Criminal record check necessary?
- Yes, if done regularly this is regulated activity. Requires enhanced DBS and barred list check.
7.3.4.2 Occupational health clearance necessary?
- Yes, if there is direct contact.
7.3.4.3 LOA or HRC?
- HRC.
7.3.5 Researcher has opportunity for any form of contact with children in the same children’s hospital (formerly a specified place) but is not providing healthcare or other types of regulated activity and has no direct bearing on the quality of care
7.3.5.1 Criminal record check necessary?
- Yes, if done regularly enhanced DBS (Pre-September 2012 definition). No barred list check.
7.3.5.2 Occupational health clearance necessary?
- Yes, if there is direct contact.
7.3.5.3 LOA or HRC?
- LoA.
7.3.6 Researcher has access to persons in receipt of healthcare services in the course of their normal duties but is not providing health care or other types of regulated activity and has no direct bearing on the quality of care (access relates to where individuals will have physical, direct contact with patients for example, observation, qualitative interviews, focus groups)
7.3.6.1 Criminal record check necessary?
- Yes, standard.
7.3.6.2 Occupational health clearance necessary?
- Yes, if there is direct contact.
7.3.6.3 LOA or HRC?
- LoA.
7.3.7 Researcher has indirect contact with patients or service users but is not providing healthcare or other types of regulated activity and has no direct bearing on the quality of care (for example, some types of telephone interview)
7.3.7.1 Criminal record check necessary?
- No.
7.3.7.2 Occupational health clearance necessary?
- No.
7.3.7.3 LOA or HRC?
- LoA.
7.3.8 Researcher requires access to identifiable patient data derived from health records, tissues or organs with a likely direct bearing on the quality of care.
7.3.8.1 Criminal record check necessary?
- No.
7.3.8.2 Occupational health clearance necessary?
- Yes, only if working with tissues or organs in NHS facilities.
7.3.8.3 LOA or HRC?
- LoA.
7.3.9 Researcher requires access to identifiable patient data derived from health records, tissues or organs with no direct bearing on the quality of care
7.3.9.1 Criminal record check necessary?
- No.
7.3.9.2 Occupational health clearance necessary?
- Yes, only if working with tissues or organs in NHS facilities.
7.3.9.3 LOA or HRC?
- LoA (only if reviewed in NHS facilities)
7.3.10 Researcher requires access to anonymised patient data derived from health records, tissues or organs only (including by research staff analysing data)
7.3.10.1 Criminal record check necessary?
- No.
7.3.10.2 Occupational health clearance necessary?
- Yes, only if working with tissues or organs in NHS facilities.
7.3.10.3 LOA or HRC?
- None.
7.3.11 Researcher is working on NHS premises (for example, laboratory) only (no access to identifiable data)
7.3.11.1 Criminal record check necessary?
- No.
7.3.11.2 Occupational health clearance necessary?
- Yes, only if working with tissues or organs in NHS facilities.
7.3.11.3 LOA or HRC?
- LoA.
7.3.12 Researcher requires direct contact with staff only but no access to patients (for example, staff interviews)
7.3.12.1 Criminal record check necessary?
- No.
7.3.12.2 Occupational health clearance necessary?
- No.
7.3.12.3 LOA or HRC?
- LoA (if in NHS facilities)
7.3.13 Researcher requires access to identifiable staff data only
7.3.13.1 Criminal record check necessary?
- No.
7.3.13.2 Occupational health clearance necessary?
- No.
7.3.13.3 LOA or HRC?
- LoA (if in NHS facilities)
7.3.14 Researcher requires access to anonymised staff data only
7.3.14.1 Criminal record check necessary?
- No.
7.3.14.2 Occupational health clearance necessary?
- No.
7.3.14.3 LOA or HRC?
- LoA (if in NHS facilities)
7.3.15
7.3.15.1 Criminal record check necessary?
- No.
7.3.15.2 Occupational health clearance necessary?
- No.
7.3.15.3 LOA or HRC?
- LoA (if in NHS facilities)
7.4 Appendix D List of abbreviations
| Abbreviation | Long form |
|---|---|
| DHSC | Department of Health and Social Care |
| RP | Research passport |
| HRC | Honorary research contract |
| LoA | Letter of access |
| HCC | Honorary collaborative contract |
| NIHR | Nation Institute of Health Research |
| HR | The People Experience team |
| HEI | Higher education institutes |
| UKCPC | UK Clinical Research Collaboration |
| HRA | Health Research Authority |
| IRAS | Integrated research application system |
Document control
- Version: 2.2.
- Unique reference number: 492.
- Ratified by: Corporate policy approval group.
- Date ratified: 29 January 2024.
- Name of originator or author: Research governance manager.
- Name of responsible individual: Research panel.
- Date issued: 12 February 2024.
- Review date: April 2025.
- Target audience: All staff involved in research.
Page last reviewed: April 15, 2024
Next review due: April 15, 2025